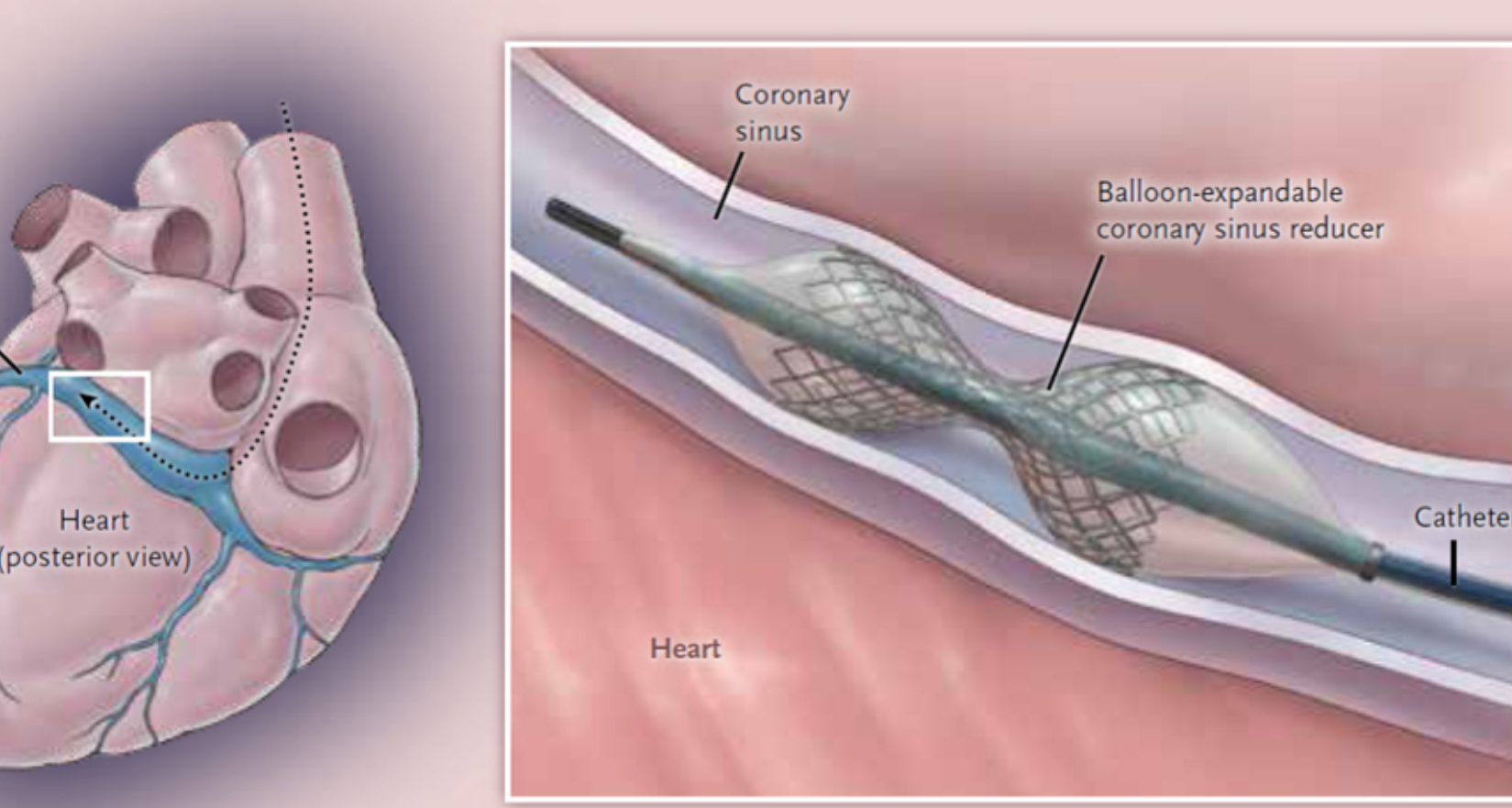
The University of Arizona Sarver Heart Center will participate in a groundbreaking trial (COSIRA II) in the United States, focused on the safety and efficacy of a device for patients with refractory angina pectoris, a medical condition that causes chest pain due to a lack of sufficient blood supply to the heart.
The device, titled Coronary Sinus Reducer TM (Neovasc Inc, Minneapolis, MN), is an hourglass-shaped stent placed in the coronary sinus (the venous system of the heart). During the randomized sham trial, which is considered the best quality trial for devices, all participants will undergo the same process in the cardiac catheterization laboratory, but only half will receive the device, through a neck vein access point with device implanted in the coronary sinus of the heart. This means the patient and the physician tracking their results do not know if they have the device implanted in them or not. Michel Corban, MD, the principal investigator for the trial, states this process ensures evaluations of the patients’ change in symptoms and quality of life are objective.
380 patients are needed to participate in the trial. Each participant will be followed up for a year. To qualify, patients will need to have obstructive coronary artery disease (blockages in the big fat vessels of the heart) and are unqualified for stents and/or surgery. Patients with obstructive artery disease may also qualify if they are considered too high of a risk for bypass surgery or a stent.
“Currently, this patient population only has one option, which is medical therapy, and if medical therapy doesn’t work, then there is nothing else we can offer,” said Dr. Corban. There are four classes of medication therapies. Patients need to be taking at least three of the classes of medications with persistent symptoms to qualify to participate in the study.
 The Food and Drug Administration has previously granted a breakthrough designation for the device. According to the FDA, this designation is for certain medical devices that provide for more effective treatment of life-threatening or irreversibly debilitating diseases or conditions. The goal is to provide patients and health care providers with timely access to these medical devices by speeding up their development, assessment, and review.
The Food and Drug Administration has previously granted a breakthrough designation for the device. According to the FDA, this designation is for certain medical devices that provide for more effective treatment of life-threatening or irreversibly debilitating diseases or conditions. The goal is to provide patients and health care providers with timely access to these medical devices by speeding up their development, assessment, and review.
The Sarver Heart Center is one of 15 current locations participating in the COSIRA-II trial, and begins recruitment in January 2023. “Collaboration is extremely important because not a single center can enroll in a timely manner the number of patients needed to power the study. If all the centers do not work together, the trial simply cannot get done," said Corban. This is the only trial addressing refractory angina with obstructive coronary artery disease and no revascularization options currently at the Sarver Heart Center.

