Most patients with heart valve disease may be candidates for the transcatheter aortic valve replacement (TAVR) procedure as an alternative to open-heart surgery. In August 2019, the Food and Drug Administration (FDA) expanded the eligibility criteria to patients who are at low risk for major complications associated with open-heart surgery. Previously the FDA approved TAVR devices only for patients evaluated as medium or high risk.
The transcather aortic valve replacement program (TAVR) started at the UArizona Sarver Heart Center in 2012. The TAVR procedure is a minimally invasive catheter-based treatment option to replace aortic valves affected by severe aortic stenosis. In this condition, the valve does not open properly and blood cannot leave the heart chambers to get out to the rest of the body. Most patients return home the following day and recover more quickly than patients who undergo open-heart surgery.

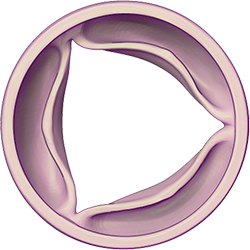
Illustrations: Healthy valve closed and opened to allow blood to leave the heart chambers and return to the rest of the body

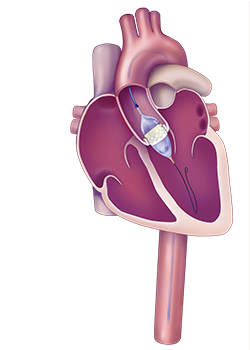

During the TAVR procedure, the inteventional cardiologist feeds a valve in a tube through the femoral artery. Once in place, the tube is expanded and the replacement valve is set in place.
For more information or to refer a patient, contact:
Structural Heart Disease Team
Phone number: 520-694-6190 • Fax: (520) 694-1007
Locations: Banner - University Medicine North and procedures at Banner - University Medical Center Tucson.

