
Personalized medicine in a dish. A collaborative research team is searching for genetic themes among family members who share a high prevalence of bicuspid aortic valve disease. They are testing medical therapies on cellular models grown in the lab from stem cells derived from each family member. Their goal is to more precisely predict which therapies would manage the conditions effectively. From left: Jennifer Andrews, PhD, assistant professor of pediatrics; Matthew Kern, second-year medical student; Jared Churko, PhD (holding a 3-D model), assistant professor of cellular and molecular medicine; and Scott Klewer, MD, professor of pediatrics, medicine, and cellular and molecular medicine.
A family with a desire to help others is giving University of Arizona Sarver Heart Center physicians and scientists an opportunity to learn more about the genetics of bicuspid aortic valve (BAV) disease.
BAV affects about 2% of the entire population, according to the Journal of American College of Cardiology. “If a pediatrician sees 50 patients a day, most likely one would have BAV,” said Scott Klewer, MD, professor of pediatrics, medicine and cellular and molecular medicine at the UA 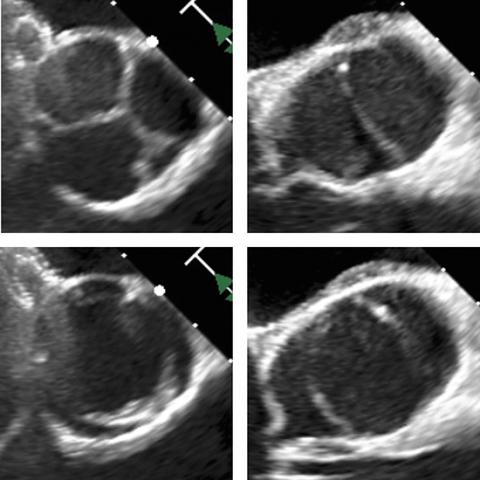
BAV typically causes obstruction to blood flow leaving the left side of the heart to the body. While most often an isolated finding, some families are impacted by a hereditary form of BAV, where 25 to 50% of all family members can be affected. Some families may have variations in the severity of BAV disease. A two-generation family under the care of Dr. Klewer has experienced this directly — two of their three children have a form of BAV.
“I follow the son and daughter at the Children’s Clinics for Rehabilitative Services and the father at Banner Adult Congenital Heart Clinic,” Dr. Klewer said. The father and son have isolated bicuspid aortic valve disease. The daughter, however, has hypoplastic left heart syndrome (HLHS), a birth defect in which the entire left side of the heart does not form. Babies with HLHS cannot live without a series of heart surgeries during infancy.
For many living with BAV disease, dilation of the aorta is an issue that develops and progresses over time and can lead to catastrophic consequences due to tearing or rupture. “The big challenge for congenital cardiologists is to determine the right time and the right therapy to intervene,” Dr. Klewer said. “So, now we evaluate available medical therapies without having predictive models; unfortunately, laboratory studies have not translated well to clinical trials. With these limitations in mind, personalized medicine may help us predict who will respond to a specific therapy.”
That’s when Dr. Klewer turned to the UA iPSC Core in Sarver Heart Center. Jared Churko, PhD, assistant professor of cellular and molecular medicine at the UA College of Medicine – Tucson, is director of the UA iPSC (induced pluripotent stem cells) Core.
In this family’s case, Dr. Churko’s research team drew blood from each family member and converted each individual’s cells into iPSCs. From this stage, Dr. Churko’s research team will convert iPSCs into various cardiac cell types to discover the disease mechanisms leading to this family’s heart defect. “We’ll use the iPS cells to create valve cells, model the disease in a dish and then try drug therapies to see if disease processes can be altered,” Dr. Churko said.
“We don’t know the underlying mutation that causes the defect, but we’re looking for a common variant in blood samples from the parents and the two affected children,” Dr. Churko said. The Churko lab also conducted whole genome sequencing for the family, something that was unaffordable until a few years ago. “This technology gives us a genetic roadmap to know more than we possibly could before,” Dr. Klewer said.

The research team includes Tom Doetschman, PhD, and Raymond Runyan, PhD, both professors of cellular and molecular medicine in the UA College of Medicine – Tucson, David Bull, MD, professor of cardiothoracic surgery, Sydney Pettygrove, PhD, assistant professor of epidemiology and biostatistics in the Mel and Enid Zuckerman College of Public Health, Michael Seckeler, MD, associate professor of pediatrics, Jennifer Andrews, PhD, assistant professor of pediatrics, and Matthew Kern, second-year UA medical student.
The research team is looking for a genetic theme based on the family members’ conditions and seeking new insights to contribute to this field of knowledge.
The Promise of Human iPS Cells
Repairing heart muscle damaged by a heart attack or other cardiovascular diseases is one of the “holy grails” for cardiovascular scientists. The ability to repair heart muscle — especially by using a person’s own cells— would 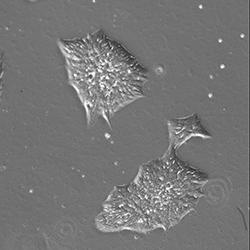
Researchers believe human-induced pluripotent stem cells (hiPSC) may be the key to unlocking the cardiomyocytes’ (heart muscle cells) regenerative ability. This research, however, is in its infancy and the technique is not ready to be deployed for human heart disease regenerative purposes.
Other Health Conditions Under Study
For another research project, the Churko lab is using funds from the Steven M. Gootter Foundation Investigator Award to create a model to study arrhythmogenic cardiomyopathy (ACM), a condition that is found in 22% of sudden cardiac deaths. Although ACM is considered a genetic disease, exercise and other contributing non-genetic environmental factors are suspected to increase risks. However, researchers still do not understand completely the mechanisms leading to ACM.
“We have collected blood samples from ACM patients and are working on a model to assess arrhythmias in cells while under stress. We hope to prove our model works and use data from this pilot research to apply for national funding to study ACM on a larger scale,” Dr. Churko said.
In addition to support from the UA Sarver Heart Center, the UA iPSC Core is supported by the BIO5 Institute and the UA Center for Innovation in Brain Science.

